Tildacerfont:Mechanism, Bioactivity and Chemical and Toxicological Studies
General description
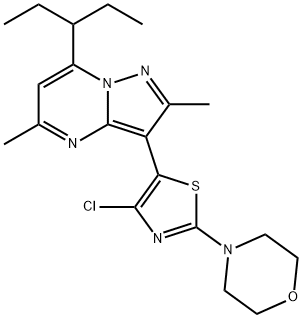
The tildacerfont, with the CAS No:1014983-00-6, is also known as 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-7-(l-ethyl-propyl)-2,5-dimethylpyrazolo [1,5-a]pyrimidine. It belongs to thiazolyl-pyrazolopyrimidine compound. This chemical’s molecular formula is C20H26ClN5OS and molecular weight is 419.97. It is a corticotropin-releasing factor type-1 receptor antagonist, may reduce excess androgen production, allowing for glucocorticoid (GC) dose reduction.[1]
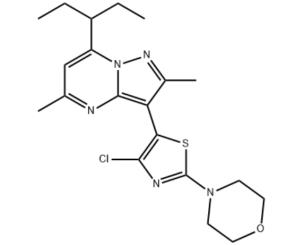
Figure 1 the molecular formula of Tildacerfont
Pharmacodynamics
Congenital adrenal hyperplasia (CAH) is a group ofrare inherited autosomal recessive disorders characterized by a deficiency of one of the enzymes needed to make specific hormones. CAH affects the adrenal glands located at the top of each kidney. Normally, the adrenal glands are responsible for producing three different hormones:(1) glucocorticoids, which gauge the bodys response to stress, illness, or injury; (2) mineralocorticoids, which regulate salt and water levels; and (3) androgens, which are male sex hormones. An enzyme deficiency may make the body unable to produce one or more of these hormones, which in turn may result in the overproduction of another type of hormone precursor in order to compensate for the loss.[5] For patients with 21-hydroxylase deficiency (21OHD), up to 12 weeks of oral tildacerfont reduced or maintained key hormone biomarkers toward normal.[1] Tildacerfont, have been trialled in poorly controlled 21OHD-CAH patients, and both reduced ACTH and androgen biomarkers while patients were on stable glucocorticoid replacement.[2]
Mechanism of action
Congenital adrenal hyperplasia due to 21-hydroxylase deficiency (21OHD) is typically treated with lifelong supraphysiologic doses of glucocorticoids (GCs). Tildacerfont, a corticotropin-releasing factor type-1 receptor antagonist, may reduce excess androgen production, allowing for GC dose reduction.[1]
Preparation
First Preparation:Under a nitrogen atmosphere dissolve 3-(4-bromo-2-morpholin-4-yl-thiazol-5-yl)-7-(l-ethyl-propyl)-2,5-dimethyl-pyrazolo[l,5-a]pyrimidine (116mg, 0.25mmol) in THF 25 (1.5mL) and chill to -78℃. Add n-butyl lithium (0.1mL. 2.5 M in hexane, 0.25mmol)and stir at -78℃for 30 min. Add N-chlorosuccinimide (33.4mg, 0.25mmol) and stir for another 30 min, slowly warming to room temperature. After stirring overnight,quench the reaction by adding a solution of saturated ammonia chloride and extract with ethyl acetate. Wash the organic layer with brine, dry over sodium sulfate, filter, and concentrate to a residue. Purify the crude material by flash chromatography, eluting with hexanes: dichloromethane: ethyl acetate (5:5:2) to provide the title compound (54mg).[3]
Second Preparation:Combine 7-(l-ethyl-propyl)-3-iodo-2,5-dimethyl-pyrazolo[l,5-a]pyrimidine (9g, 26.2mmol) and 4-chloro-2-morpholino-thiazole (7.5g, 36.7mmol) in dimethylformamide (90mL) previously degassed with nitrogen. Add cesium carbonate (17.8g, 55mmol), copper iodide (250mg, 1.31mmol), triphenylphosphine (550mg, 2.09mmol) and palladium acetate (117mg, 0.52mmol). Heat the mixture to 125℃ for 16 hand then cool to 22°C. Add water (900mL) and extract with methyl-/-butyl ether (3 x 200mL). Combine the organic portions and evaporate the solvent. Purify by silica gel chromatography eluting with hexanes/ethyl acetate (4/1) to afford the title compound (6.4g, 62%).[3]
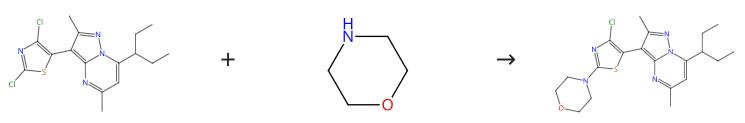
Third Preparation:Charge7-(l-ethyl-propyl)-3-(2,4-dichloro-thiazol-5-yl)-2,5-dimethylpyrazolo[l,5-a]pyrimidine (0.37g, 1.00mmoles), K2CO3 (0.28g, 2.0mmoles) and anhydrous morpholine (3mL) to a round bottom flask equipped with a magnetic stir bar and N2 inlet. Stir the yellow mixture at 100℃ for about 4 hr, during which time the reaction becomes homogeneous. Cool the reaction mixture to room temperature, add H2O (10mL) and stir the heterogeneous reaction mixture overnight at room temperature. Collected the yellow solid by fdtration, wash with H2O and allowed to air dry overnight to give the crude title compound (391mg). Recrystallize from isopropyl alcohol (3mL) to yield the title compound as a light yellow crystalline solid (380mg, 90.6% yield, 99% by LC).[4]
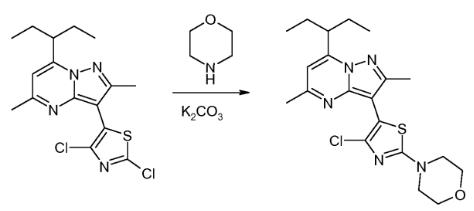
Figure 2 Preparation of Tildacerfont
Fourth Preparation: 7-(l-ethyl-propyl)-3-(2,4-dichloro-thiazol-5-yl)-2,5-dimethyl-pyrazolo[l,5- a]pyrimidine (15.2g, 41.16mmoles) is charged into a 250mL 3-necked round bottomed flask, followed by addition of 2-MeTHF (61mL, 4.0volumes), the yellowish brown slurry is stirred at about 20℃ for 5 min. Then morpholine (19g, 218.18mmoles) is added over 2-5 minutes. Contents are heated to reflux and maintained at reflux for 12 hr. The slurry is cooled to 25°C, followed by addition of 2-MeTHF (53mL, 3.5 volumes) and water ( 38mL 2.5volumes). The reaction mixture is warmed to 40°C, where upon a homogenous solution with two distinct layers formed. The layers are separated, the organic layer is fdtered and concentrated to ~3 volumes at atmospheric pressure. Four volumes 2-propanol (61mL) are added. The solution is concentrated to ~3 volumes followed by addition of 4 volumes 2-propanol (61mL), re-concentrated to ~3 volumes, followed by addition of another 6 volumes 2-propanol (91mL), and refluxed for 15 min. The clear solution is gradually cooled to 75℃, seeded with 0.45g 7-(l-ethyl-propyl)-3-(4-chloro-2-morpholin-4-yl-thiazol-5-yl)-2,5-dimethyl-pyrazolo[l,5-a]pyrimidine slurried in 2mL 2-propanol, rinsed with an additional 2mL 2-propanol and transferred to a crystallization flask. The slurry is cooled to between 0-5°C, maintained for I hr, fdtered and the product rinsed with 2-propanol (30mL, 2volumes). The solid is dried at 60℃ in a vacuum oven to afford 16.92g 7-(l-ethyl-propyl)-3-(4-chloro-2-morpholin-4-yl-thiazol-5-yl)-2,5-dimethyl-pyrazolo[l,5-a]pyrimidine. Purity of tildacerfont by HPLC assay is 100.00 %.[4]
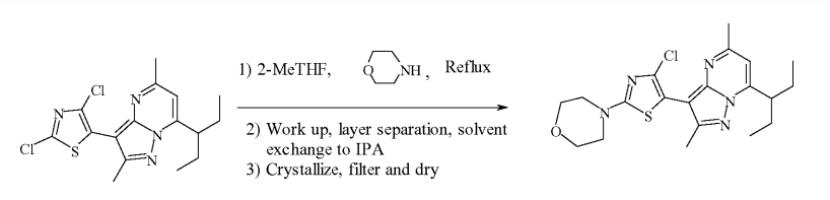
Figure3 Preparation of Tildacerfont
Toxicity
A study that tildacerfont in adults with classic congenital adrenal hyperplasia show results from two phase 2 studies safety was evaluated using adverse events (AEs) and laboratory assessments: AEs (in 53.6% of patients overall) included headache (7.1%) and upper respiratory tract infection (7.1%).[3]
References
[1]Kyriakie S, Barnes CN, Huang M, et al. Tildacerfont in Adults with Classic Congenital Adrenal Hyperplasia: Results from Two Phase 2 Studies[J]. The Journal of Clinical Endocrinology; Metabolism, 2021.
[2]Prete A,Auchus RJ, Ross RJ. Clinical advances in the pharmacotherapy of congenital adrenal hyperplasia[J].European Journal of Endocrinology, 2022,186(1),R1-R14.
[3]Chen Z, Hamdouchi CH,Hembre EJ, et al. Thiazolylpyrazolopyrimidines as CRF1 receptor antagonists and their preparation, pharmaceutical compositions and use in the treatment of diseases[P]. 2008,WO2008036579,A120080327.
[4]Rizzo JR, Boini S Kr, Vaid RK. Thiazolyl-pyrazolopyrimidine compounds as synthetic intermediates and related synthetic processes[P]. 2010, WO2010039678, A120100408.
[5]Howerton A, Gerber H. Methods for treating testicular and ovarian adrenal rest tumors[P]. 2019, WO2019210266,A120191031.